THIS SITE IS FOR U.S. HEALTHCARE PROFESSIONALS ONLY
Second-Line (2L+) Maintenance Treatment of Recurrent Ovarian Cancer
SELECT INDICATION

Primary endpoint: Investigator-assessed PFS (N=196)b
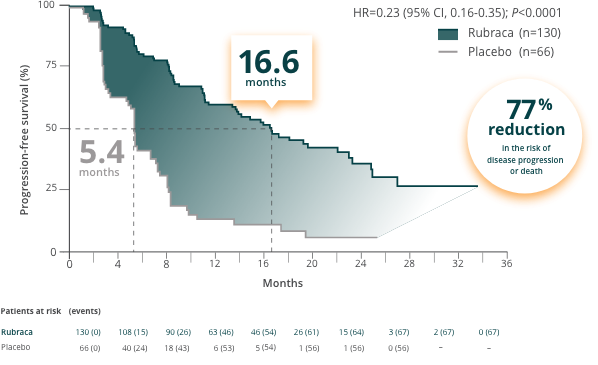
Figure references.1-3,5,6
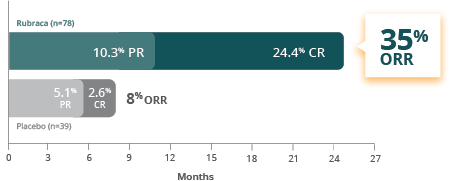
Confirmed response rate
Primary endpoint: Investigator-assessed PFS (N=196)b

Figure references.1-3,5,6
Confirmed response rate

The information contained in this website is intended for U.S. healthcare professionals only.
By clicking the button below, you acknowledge that you are a U.S. healthcare professional.