THIS SITE IS FOR U.S. HEALTHCARE PROFESSIONALS ONLY
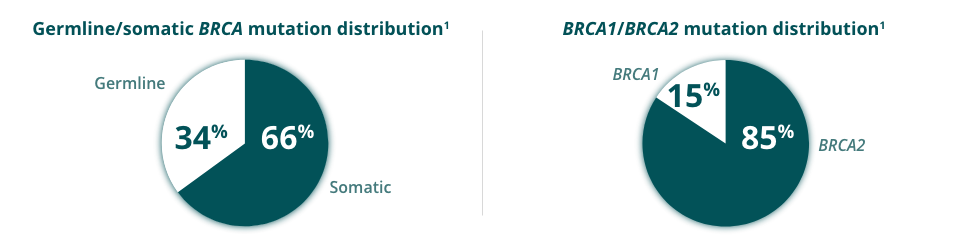
BRCAmut+ Metastatic Castration-Resistant Prostate Cancer (mCRPC)
SELECT INDICATION




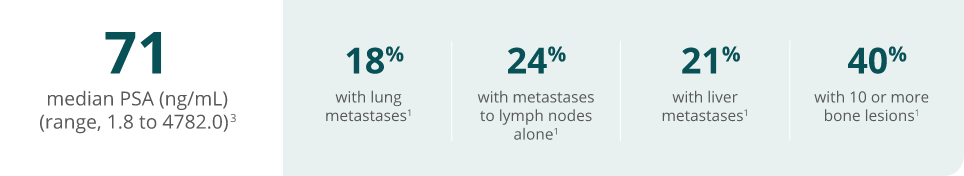
Changes in PSA levels may be observed but have not been shown to correlate with clinical
benefit or progression in individual patients treated with Rubraca

Continue until disease progression or unacceptable toxicity.1
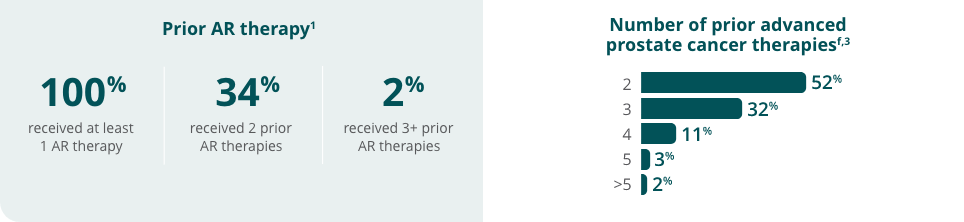
All patients received a concomitant GnRH analog or had prior bilateral orchiectomy.1


The information contained in this website is intended for U.S. healthcare professionals only.
By clicking the button below, you acknowledge that you are a U.S. healthcare professional.