Continue until disease progression or unacceptable toxicity1
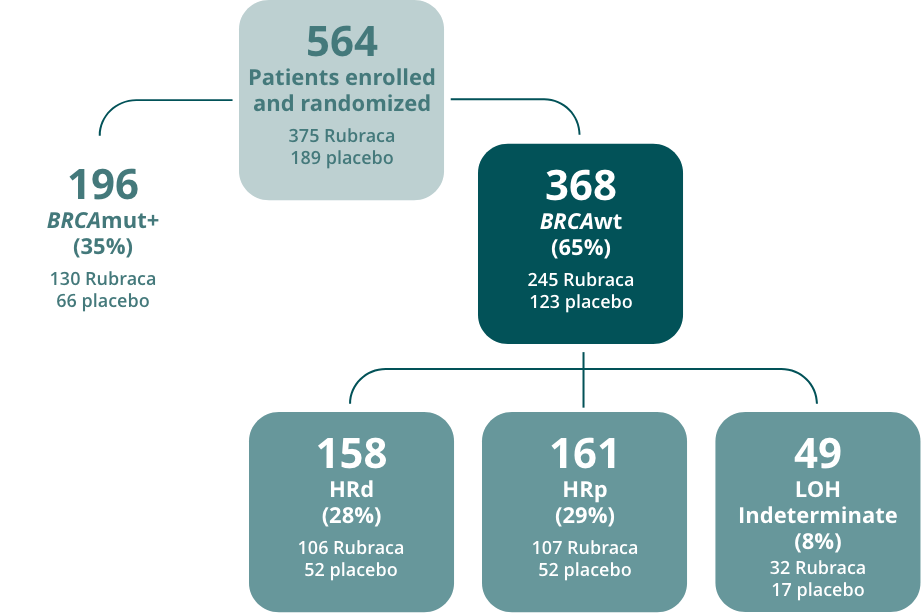
Randomized patients evaluated per a step-down analysis of 3 nested cohorts.b,1,2



Continue until disease progression or unacceptable toxicity1
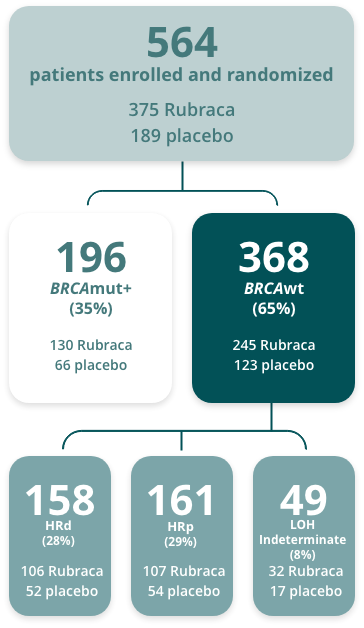
Randomized patients evaluated per a step-down analysis of 3 nested cohorts.b,1,2


The information contained in this website is intended for U.S. healthcare professionals only.
By clicking the button below, you acknowledge that you are a U.S. healthcare professional.